In this article, we'll explain a core concept of diving: partial pressures. When we are diving, one of the important things we want to keep track of is the gas we are breathing: partial pressures help us define a relation between the gas blend that we breathe, and each individual component of that blend (oxygen, nitrogen, helium, etc...). Let's take a dive into partial pressures!
Definition
A mixture of gas (or a gas blend), which is made up of multiple gases, will exert a certain pressure (in the context of scuba-diving, that pressure will vary depending on the depth the diver finds himself at): we can see the partial pressure of each gas as "components" of the total pressure exerted by the mixture. This means that the total pressure is the sum of each individual pressure
Dalton's law
Dalton's law states that the sum of all partial gases in an ideal gas mixture is equal to the total pressure of that mixture. The image below depicts an example of Dalton's law in a gas mixture containing two separate gases.
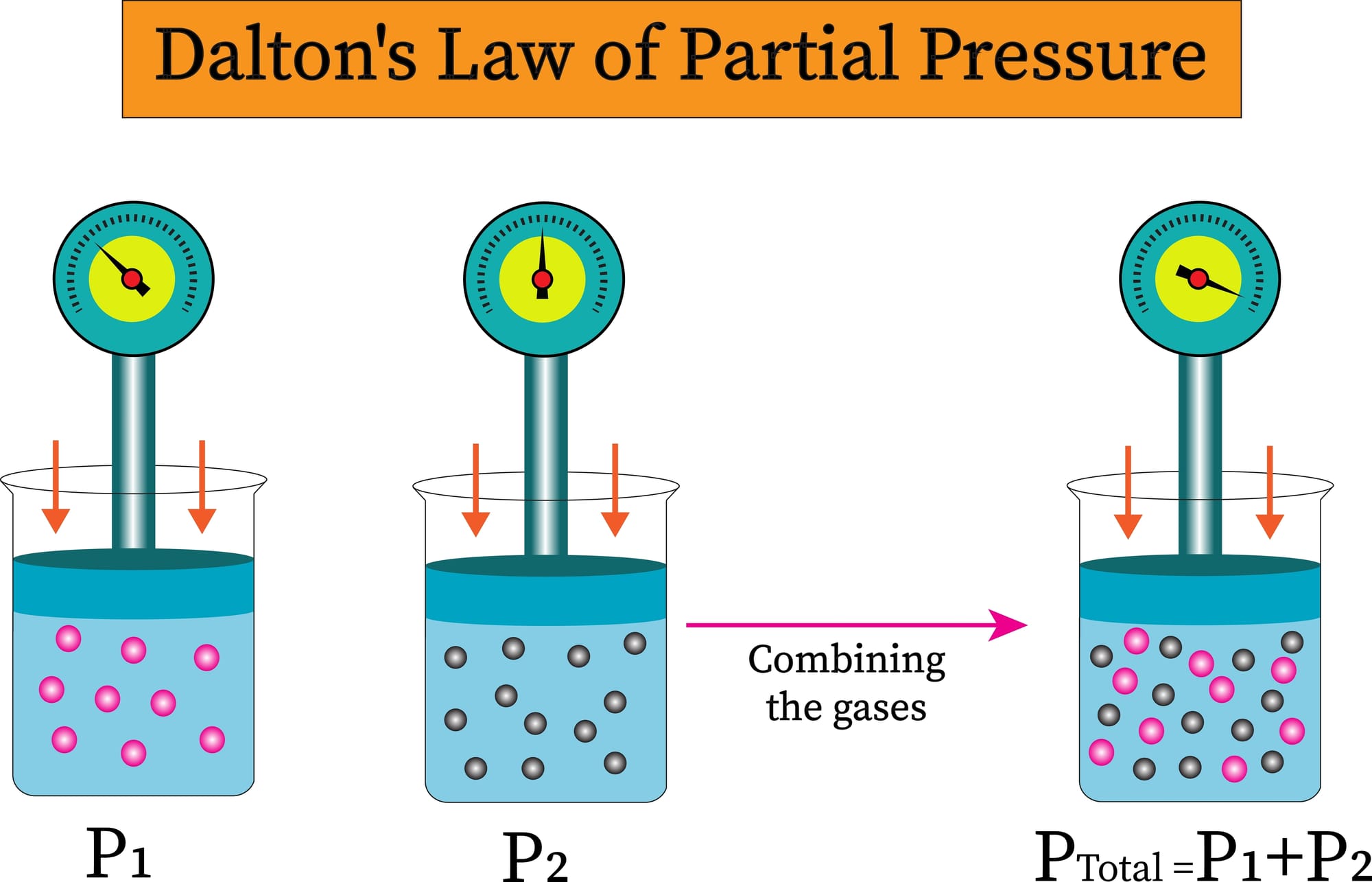
We can generalize this law to a gas mixture containing n ideal gases:
\( P_{tot} = P_{1} + P_{2} + \cdots + P_{n} = \sum_{i=1}^{n} P_{i} \)When diving (although it is a simplification), we usually only consider the constituents of air to be Oxygen and Nitrogen. We could then write:
\( P_{tot} = P_{O_2} + P_{N_2}\)If we use a trimix blend (a mix of oxygen, nitrogen, and helium, which technical divers use to reduce the narcotic potential of the mix), we would have:
\( P_{tot} = P_{O_2} + P_{N_2} + P_{He}\)Using this additive relationship, we can figure out the partial pressure of a gas: for example, in a nitrogen-oxygen blend which has a total pressure of 1 atm, if the partial pressure of oxygen in the mix is 0.3 atm, we can figure out that the partial pressure of nitrogen must be 0.7 atm.
There is another way of finding the partial pressure of a gas if we know what the proportion of the gas in the mixture: the partial pressure of the gas is proportional to the proportion of the gas, and therefore, for a gas i, we can calculate:
\( P_{i} = P_{tot} \cdot F_{i}\) \( P_{i}: \text{Partial pressure of the gas}\) \( F_{i}: \text{gas fraction (expressed between 0 and 1)}\)This allows us to calculate the partial pressure of a gas as the total pressure increases when the diver descends. For example, if a diver were breathing air at a depth of 20 meters, he would be subjected to a total pressure of 3 atm. We could then find the inhaled oxygen partial pressure: pO2 = 3 atm x 0.21 = 0.63 atm.
Henry's law
Henry's law states that the amount of gas that can be dissolved in a solution is proportional to the partial pressure of that gas. The reason why this law is important in the context of diving is that the deeper a diver goes, the more nitrogen will be dissolved in their blood and tissues (and of course, the longer they stay at depth, the more nitrogen will actually saturate their body as the saturation of each tissue takes time). Upon shallowing up, as the total pressure and the partial pressure of each gas in the blend diminish, the solubility diminishes as well: in other words, you can store more nitrogen in its dissolved form the deeper you are.
If the diver hasn't exceeded a certain safe threshold of nitrogen saturation in their body (usually represented by the no-decompression limit), they would be able to safely ascend to the surface with minimal risks of nitrogen coming out of solution, forming bubbles, and encountering any decompression-illness symptoms. On the other hand, if the diver stays too deep for too long and exceeds their no-decompression limit, shallowing too quickly might mean that all the dissolved nitrogen cannot stay dissolved and would form bubbles. This is the goal of decompression diving: staying at certain depths for a certain amount of time to off-gas that "excess nitrogen" before surfacing to ensure there is no bubble formation in the body.
The relevance of partial pressures in diving
The reason partial pressures are such an important topic in the world of scuba diving is that we are constantly monitoring them and choosing the gas we breathe (especially for the deeper dives) as a function of how much we want to limit our oxygen and nitrogen partial pressures. As we will see, a lot of our limiting factors have to do with the partial pressures of oxygen and nitrogen.
Hyperoxia
Hyperoxia means breathing an excessive amount of oxygen: as we go deeper, the total pressure increases, which in turn, increases the partial pressure of Oxygen. Although Oxygen keeps us alive, it turns out that in high enough amounts, it becomes toxic. As recreational divers, we ensure we never exceed an oxygen partial pressure of 1.4 atm: breathing air, the depth at which we would get an oxygen partial pressure of 1.4 atm is 56 meters. When we use nitrox, as the oxygen fraction is higher, our depth is more limited: this is why calculating the MOD (Maximum Operating Depth) of a gas is important, as it helps us keep hyperoxia under control. During technical/decompression dives, the maximum oxygen partial pressure allowed for the deco mixes (meaning the gas blends rich in oxygen, used to off-gas nitrogen faster) is 1.6 atm.
The consequences of hyperoxia can vary depending on the type of oxygen exposure. For example, a short but intense exposure to a high oxygen partial pressure will lead to CNS (Central Nervous System) Oxygen toxicity, while a longer but more moderate exposure to oxygen will lead to pulmonary and ocular oxygen toxicity.
Hypoxia
Hypoxia means that there is a lack of oxygen. This scenario happens when the partial pressure of oxygen is lower than 0.17 atm. A gas blend with a higher oxygen fraction than 17% is known as a "normoxic mix", this is because the mix can be safely breathed on the surface, while any mix with less than 18% oxygen is known as a "hypoxic mix", as it won't be breathable on the surface and the diver will need to descent to increase the partial pressure to breathe it.
This case happens for deep dives (deeper than 60 meters) when the diver has to limit their exposure to the inhaled oxygen partial pressure and therefore has to blend a gas mix with a lower oxygen fraction. This is done by adding helium into the mix, which will, in turn, reduce the oxygen and nitrogen part.
Inert gas Narcosis
Under pressure, inert gases can become narcotic. Although it is hard to draw a hard line at which depth/pressure does the narcosis "start", it is commonly accepted to say that the first effect of Nitrogen Narcosis start at an inspired Nitrogen partial pressure of ~3.16 bars, which equates to a depth of 30 meters using air. The issue with finding out the "narcotic partial pressure" of a gas is that measuring narcosis is tricky, and it affects everyone differently. On top of that, each different gas has a different narcotic potential.
When preparing deep technical dives, divers will choose how much helium they want to add to reduce the narcotic potential of the blend (as helium is less narcotic than nitrogen). This will give them a blend with a certain "Equivalent Narcotic Depth", which is the depth at which you would need to be on air to be exposed to the same nitrogen partial pressure (some divers will consider the oxygen as narcotic, which will force them to add even more helium to the blend to reduce the narcotic potential).
Gas Saturation in tissues
As we talked about previously in the part about Henry's law, the partial pressures of the gases play a big role in our decompression obligations, as we track our tissues' saturation level during dives and ensure it doesn't go beyond a certain threshold (or complete decompression obligations if it does).
Simply put, the higher the partial pressure of a gas is, the more of it will get dissolved in our body (meaning in our blood and various tissues). This isn't much of an issue with oxygen, as it is metabolized by our body; Inert gases like nitrogen aren’t metabolized, so they accumulate in our tissues in proportion to the partial pressure we’re breathing. When we shallow up (ascend), the inspired ppN₂ decreases; if it becomes lower than the ppN₂ in our tissues, nitrogen will diffuse out (meaning that we off-gas).
In recreational dives, we ensure the ppN2 in our tissues stays below a certain safe limit, which allows us to ascend safely to the surface at any time. For technical/decompression dives, divers will track their supersaturation level and complete increasingly shallower decompression stops, allowing the Nitrogen to safely diffuse out of their body while staying under enough pressure, which prevents any bubbles from forming. To accelerate decompression, technical divers use decompression gas with a lower nitrogen fraction (for example, blends containing 40%, 50%, or even 100% oxygen are common, even though they require special care and training as the higher the oxygen fraction, the greater the risk of oxygen toxicity) to increase the difference in ppN2 between the tissues and the inhaled gas.
Examples
To figure out how partial pressure is and how to find it, here are a couple of examples:
A diver is breathing a Nitrox mix. He analyzed the tank and found that it contains 30% oxygen. He is planning to go to a depth of 20 meters; what oxygen and nitrogen partial pressure would he be subjected to at this depth?
To find the ppO2 and the ppN2 in this scenario, we can start by figuring out that the total pressure the diver is subjected to at this depth is 3 atm (as we have 1 atm every 10 meters plus the already existing 1 atm from the surface). Now, to find the ppO2, we can simply calculate: ppO2 = 3 atm × FO2 = 3 atm × 0.3 = 0.9 atm.
To figure out the ppN2, we can then calculate: ppN2 = Ptot - ppO2 = 3 atm - 0.9 atm = 2.1 atm.
A technical diver is using a decompression mix with an oxygen fraction of 50%, if he doesn't want to exceed a ppO2 of 1.6 bar, at what maximum depth can he use that mix?
To find the maximum depth at which the mix would reach an oxygen partial pressure of 1.6 bar, we can first find out which would be the total pressure required: Ptot = ppO2/FO2 = 1.6 bar/0.5 = 3.2 bar. Now, we need to find the depth at which we get a total pressure of 3.2 bars: we know that we increase the pressure roughly by 1 bar every 10 meters, and that we already have 1 bar on the surface. That means that we would need to go to a depth of 22 meters to get a total pressure of 3.2 bars. And this gives us the maximal depth for the mix (this is also known as the MOD or Maximum Operating Depth).


